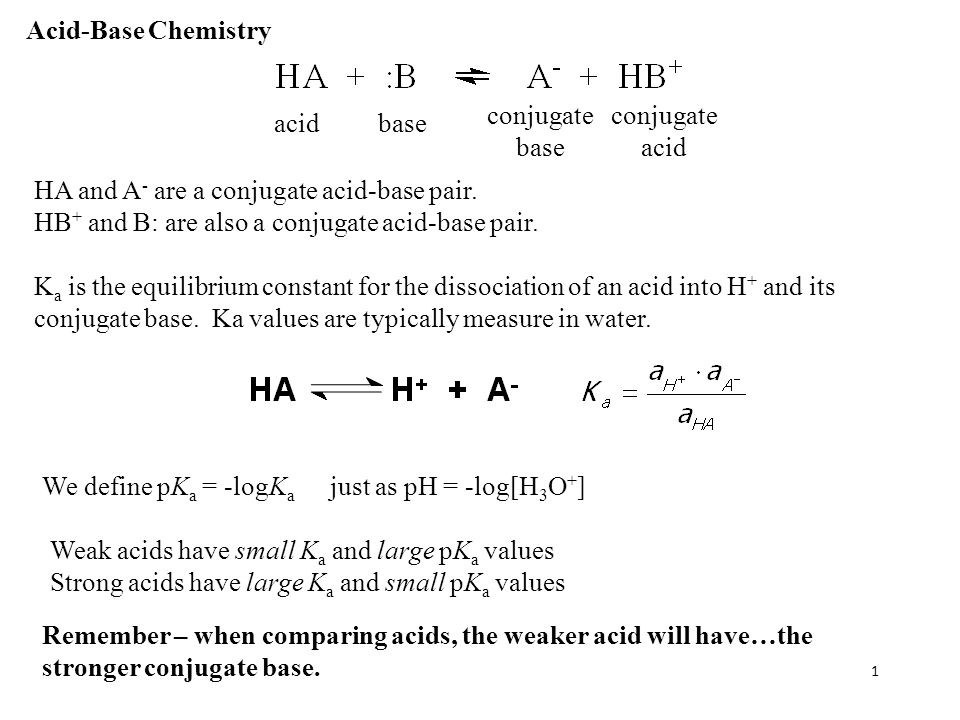
Acid-Base Chemistry K a is the equilibrium constant for the dissociation of an acid into H + and its conjugate base. Ka values are typically measure in. - ppt download

Positive regulation of the enzymatic activity of gastric H+,K+-ATPase by sialylation of its β-subunit - ScienceDirect

Question Video: Writing an Equation for the Acid Dissociation Constant of a Generic Weak Acid | Nagwa
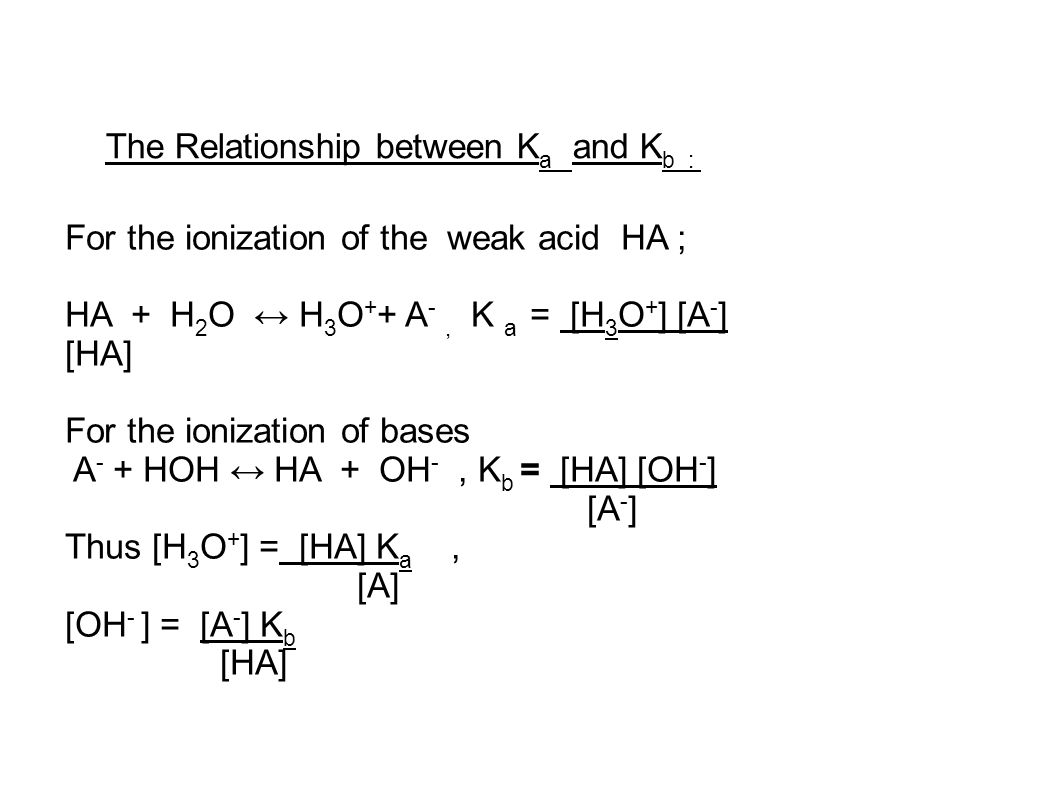


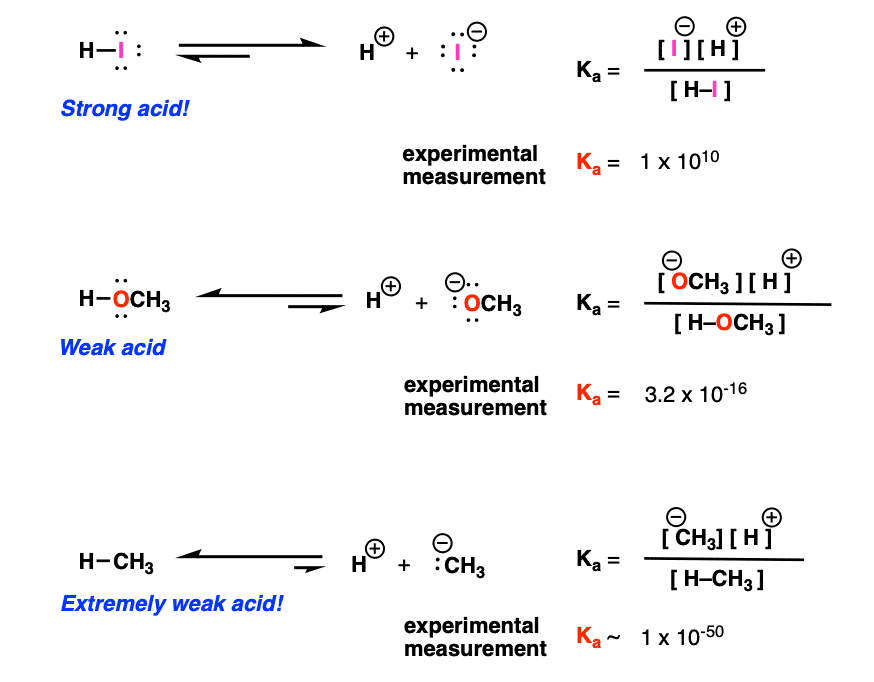






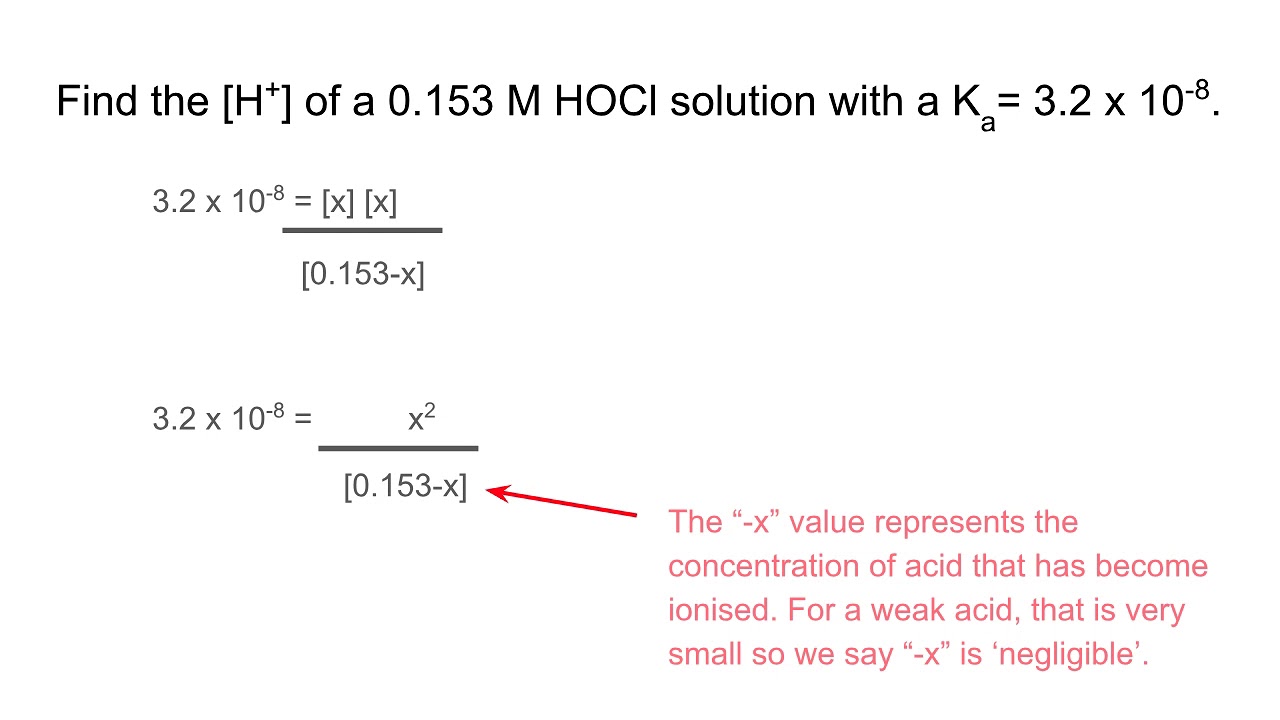


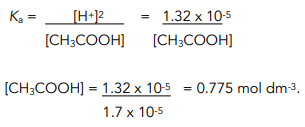
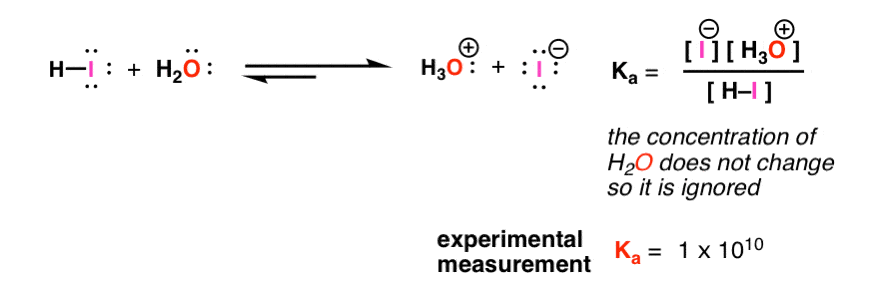
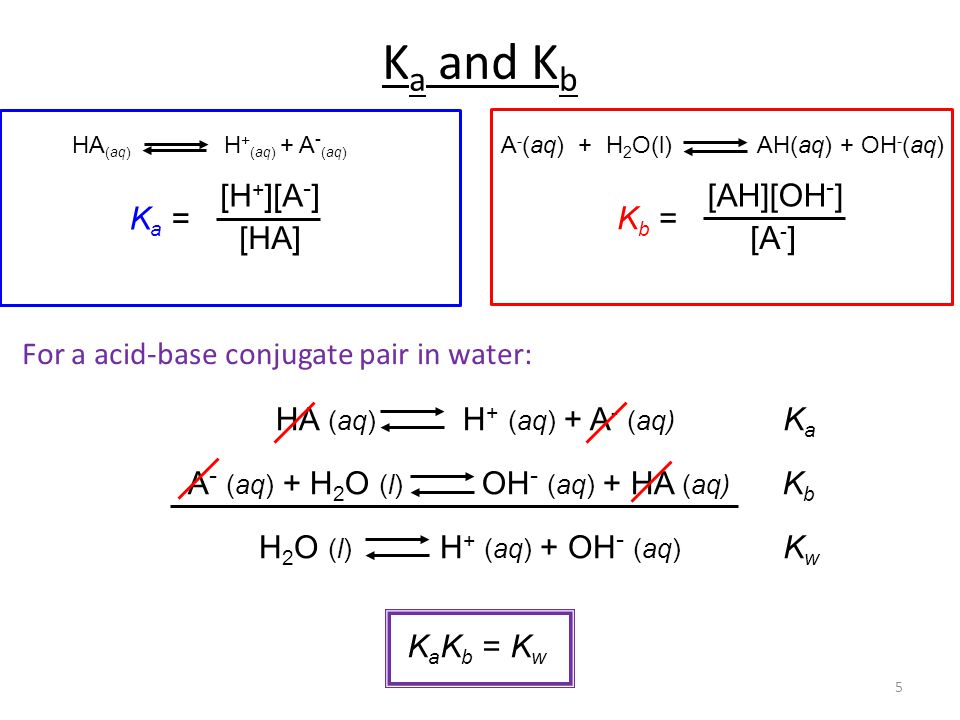
:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)
